文章
根据ASTM F2129进行医疗器械腐蚀试验
The human body and its immune and inflammatory mechanisms can create an aggressive environment for metallic implants resulting in implant rejection and failure (Gilbert, Corrosion, 2017;73(12):1478-1495). In vitro testing of small implants is typically performed per ASTM F2129 to mitigate these risks. While this testing helps determine implant susceptibility to corrosion, it does not take into account the effects of cells, proteins, and other biological effects that implants will experience in vivo.
尽管有其局限性,ASTM F2129自2001年首次出版以来已经经历了几次修订,以更好地帮助医疗器械制造商评估其小型植入物的抗点蚀和缝隙腐蚀能力。本标准提供了一种在最终形状和表面处理的整个装置上进行循环(正向和反向)动电位极化测量的试验方法。
在下面的部分中,我们将介绍test methods,装置,结果and验收标准used in vitro corrosion testing of small implants.
Test Apparatus
A typical multiport electrochemical test cell is used for testing. It has inflow/outflow ports for the nitrogen gas and heated water circulation, ports for the reference, graphite counter and working/sample electrodes, and an inner chamber for the saline bath.
附加组件包括:恒电位仪、计算机、pH计、氮气罐、水泵、带紫外线灯和固化面罩的导电环氧树脂,以及用于样品附着的不锈钢丝。使用磷酸盐缓冲盐水等试剂进行浴,使用异丙醇和II型试剂级水进行样品冲洗。光学和电子扫描显微镜用于测试前和测试后的检查。
试验方法
表1提供了一个简短的术语概要;更多详情请参考ASTM F2129标准。
简单地说,植入物在37℃的盐水中保存±1°C,起始pH值为7.4±在右边显示的设置中为0.2。在植入物和参比电极之间引入一个电位,并上升到上述生理电位。击穿通常会导致电流密度的快速增加,如电位与对数电流密度的输出图所示。需要计算或估计暴露于溶液中的样品的总表面积,以便确定样品产生的电流密度。
Samples are prepared for testing and thoroughly inspected. Any preconditioning should be performed as it can impact the behavior of the implants. Conductive epoxy is used as the interface to the sample (specimen holders vary depending on the device type), connected to a potentiostat through a wire, and resistance is checked to ensure good connection. The test cell is filled with saline (minimum of 500 mL, typically 1L), heated to the target temperature, and nitrogen gas flow at 150 mL/min is initiated. Nitrogen gas flow is used to deaerate the solution and lower dissolved oxygen concentration to decrease the potential at which oxidation and reduction currents are equal.
Temperature, pH, and nitrogen gas flow time (30 min minimum) are recorded, then the device is rinsed and inserted in the test cell with the wire. Saline is added into the reference electrode holder, and the electrode is inserted at 5mm or less from the sample, but not in contact with the sample. The nitrogen purge is continued throughout the test. The open circuit test is initiated, and Er1小时后记录。然后,通过设置E以1mv/s扫描速率启动循环极化测试我and Efto Er. The cyclic polarization is closely monitored; if a current density increase larger than two decades occurs, voltage is reversed.
When the test is completed, the final temperature, pH, Er,电子b、Ef和Ep被记录下来。将样品从试验中取出并冲洗,将金属丝夹在样品上方约1英寸处,并将样品储存起来,以备进一步的试验后检查。虽然标准没有说明应测试多少样品,但我们通常看到每个表面光洁度有3-8个样品。
试验结果
记录每个样品的试验条件:氮气流动时间、初始温度、初始pH值、最终温度和最终pH值。样品试验结果通常以表格和图形格式提供。还提供了试验前后的观察结果和检验结果。
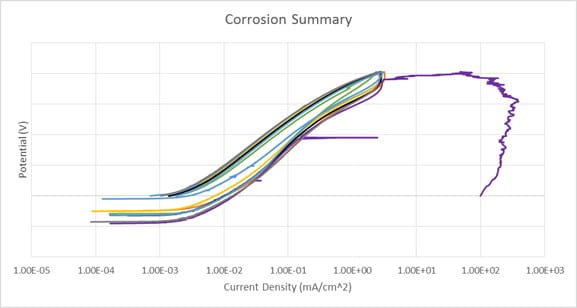
上面的图表显示了从腐蚀测试结果t of 8 samples of a nitinol device plotted as potential (mV) vs. log current (A). Results show consistency between the 8 samples; the purple data set with the increase in current density is an example of a breakdown potential.
验收标准
ASTM F2129未定义验收标准,但确定了Eb作为坑形核和生长的关键数据点。因此,E值越高b我s, the more resistant the metal is to pitting corrosion. It is also recommended that Eb将这些值与在体内表现出良好耐腐蚀性的对照装置进行比较,作为参考。设备制造商定义了植入物的验收标准。根据我们的经验,我们可以接受b值通常设置在300 mV以上,并且通常设置在更高的阈值。
Consultation
We often perform corrosion testing on medical devices to characterize their corrosion potential. If you would like more information about our医疗器械腐蚀试验services, or for assistance with your device’s corrosion and/or fatigue testing,联系我们的专家来讨论我们能帮上什么忙。
Element提供最广泛的医疗设备测试范围,因此如果您正在寻找package testing,microbiological analysis,加速老化保质期试验或EMC/EMI测试,请与我们联系以连接到正确的实验室。
Find related articles to you through the细胞核
确定了将近190年
More from Element

医疗器械
As a comprehensive testing partner, you’ll enjoy the benefit of a single supplier source for all of your testing needs, from mechanical testing and environmental simulation to EMC and wireless device testing.
阅读更多

可重复使用医疗器械验证
Our medical device experts evaluate the environmental impact on the identification markings and function of reusable medical instruments after repeated autoclave sterilization cycles.
阅读更多

医疗器械测试Tips
为医疗器械测试项目做准备对许多制造商来说都是一个挑战,尤其是当它是一个新的或新颖的产品或者时间有限的时候。我们的医疗器械专家分享他们的测试技巧和最佳实践。
阅读更多
Sign-up for free resources
访问Element的电子邮件订阅中心以接收最新的行业新闻、技术白皮书、案例研究、网络研讨会和即将到来的活动。
阅读更多

